
The expertise of our scientific and technical staff is also offered to researchers from public and private research centers and also to professionals from industrial sectors that require the use of this instrument.
Image (resolution 1.4 nm)/ Analysis:
By using the different detectors available within this instrument, the following information can be obtained:
-
Image with secondary electrons and topography by means of an ETD/TLD (Everhart-Thornley/ Thru-the-Lens Detectors).
-
Image (back scattered electrons) and composition by using a BSED (Back Scattering Electron Detector).
-
Image with secondary ions, sensitive to crystallographic direction.
-
Elementary Chemical Analysis by EDX (Energy-Dispersive X-ray micro-analysis).
-
STEM (scanning-transmission) images.
Nanofabrication (lateral dimension between 50 nm and tens of microns)
-
FIB: focused ion beam; etching of a predesigned motif over the sample.
-
FEBID/FIBID: focused electron/ion beam induced deposition.
PRECURSOR GASES
(CH3)3(CpCH3)Pt, Co2(CO)8, W(CO)6, TEOS + H2O –> SiO2, Selective Carbon Mill (MgSO4·7H20)
Micromanipulation
-
Lamellae preparation in conventional mode.
-
Thinning at low temperatures (samples for TEM observation).
-
Nano-manipulator (Omniprobe).
Low Temperature
-
Fast freezing and cryo-fracture of materials. Samples can be fractured in the -180 to -150 ºC range. The observation can be made between -130 and -140 ºC ± 1 ºC.
- Non-conductive samples need metallization, which can also be done in our Centre.
- Conductive and non-conductive samples as bulk, films, powder (compacted), etc. can be studied.
- Samples should be compatible with high vacuum conditions.
- Samples in the 1 mm to 100 mm range can be studied. They should be less than 10 mm thick.
- Use of the cryo-option allows the measurement of liquid samples, semi-liquids and beam sensitive samples, polymers, resins, MOFs (metal-organic frameworks), etc.
Electron beam resolution |
2.5 nm at 1 kV, 1.4 nm at 15 kV |
Ion beam resolution |
7 nm at 30 kV |
Landing Voltage Range |
E-beam: 200 V-30 kVI-beam 2kV-30kV |
Probe current |
E-beam 1.4 pA (1kV) up to 37 nA (30kV)I-beam: 1 pA up to 20 nA at 30 kV |
High Precision5-axesmotorized stage |
XY: 50 mmZ: 25 mmT: -10 to +60R= 360° (continuous) |
Chambervacuum |
<2.6 x 10-6 mbar (after 24 h pumping) |
Sample Size |
Maximum size: 150 mm diameter with full rotation( larger samples possible with limited rotation).Weight: max 500 g (including the sample holder). |
Cryo-optionModel PPT2000 with Cryo-transfer from Quorum Technologies |
|
Transfer |
After freezing and vacuum transfer the sample is placed on the preparation chamber cold stage. Stage temperature is normally set to between -130oC and -140oC (precisely controlled to with +or- 1C) |
Fracturing |
Sample can be fractured using either the cooled probe or cryo knife Tools. |
SUBLIMATION (ETCHING): |
Water (ice) can be sublimed (etched) from the sample by raising the stage temperature (typically to between -80oC and -100oC. |
COATING |
Sample is sputter coated with Pt or C and then transferred in to the SEM cold stage. |
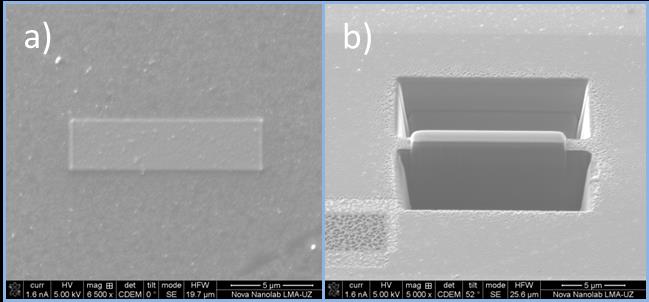
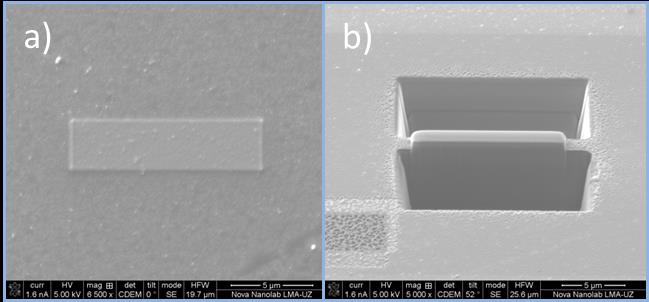
a) Pt deposited by e- over multilayer organic magnetic tunnel junctions b) View of milling by i+ beam during the process to make lamella previous to the lift out.
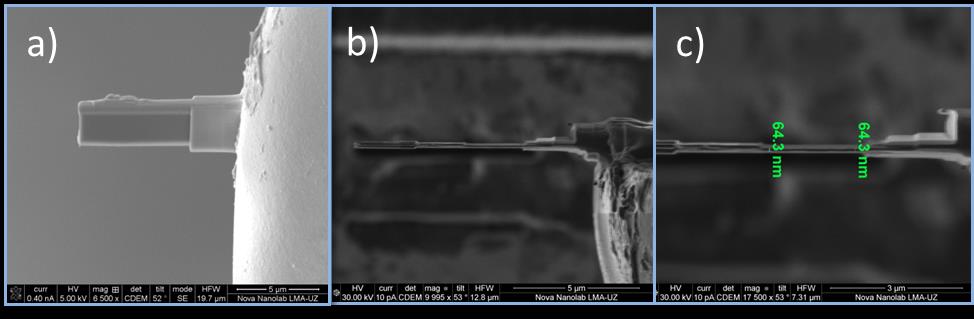
a) Electron image of lamella of organic magnetic tunnel junctions on the grid. b) Final view of the lamella, ion image c) details and thickness of lamella by ion image, the final process was made at low temperatures (-160 ºC)
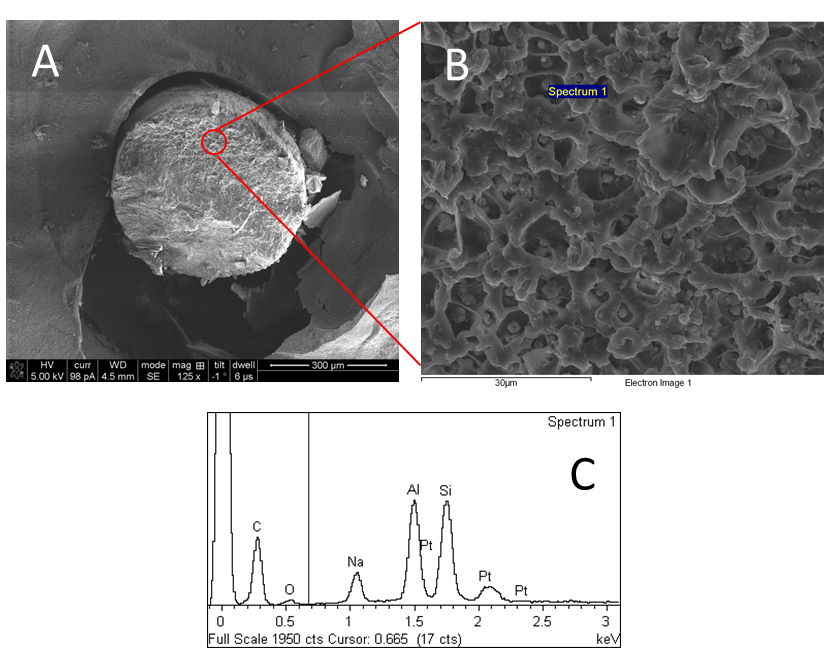
(A) General overview of polymers after cryofracture. (B) Details about the internal structure and distribution of Zeolite. (C) Show the spectral analysis of specific zeolite areas, demonstrating the presence of the aluminum and Silicon typical in the zeolite.


Laboratorio de Microscopías Avanzadas
We are a unique initiative at national and international levels. We provide the scientific and industrial community with the most advanced infrastructures in Nanofabrication, Local Probe and Electron Microscopies for the observation, characterization, nanopatterning and handling of materials at atomic and molecular scale.
Contact information
Campus Río Ebro, Edificio Edificio I+D+i
Direct Links
© 2021 LMA | Website developed by o10media | Política de privacidad | Aviso legal | Condiciones de uso | Política de Cookies |






